Part:BBa_K4155002
Added by XHD-Wuhan-Pro-China
Validation of Zinc Sensitive Promoters
We used molecular biology tools to construct plasmids pUC19 and pETDuet-1, and useda direct DNA synthesis method to synthesize zinc responsive promoter (pzntR) and riboflavin synthesis gene (ribB).
Firstly, pzntR was linked to Green fluorescent protein(GFP), and the linked gene fragment was double digested with HindⅢ and BamHⅠ, and then inserted into plasmid pUC19. The recombinant plasmid, named pUC19-pzntR-GFP, was introduced into E.coli BL21 and expressed.
Engineered E.coli BL21 strains and wild-type E.coli BL21 strains were cultured in LB liquid medium at 37℃, and different concentrations (0, 30, 60, and 90 μM) of Zn2+ were added when OD (bacterial density value) 600 was 0.6. Three hours after the addition of Zn2+, engineered and wild-type cells induced by Zn2+ were observed under a fluorescence microscope. The 96 Well Plate Reader was used to measure GFP (excitation wavelength of GFP was 488nm, emission wavelength was 507nm).
The engineered bacteria containing the zinc promoter plasmid in the left panel express green fluorescence, while the wild bacteria in the right panel do not. This suggests that zinc-sensitive promoters can be primed.
With the increase of zinc ion concentration, the absorption intensity of wild bacteria did not change and was maintained at 10. However, with the increase of zinc ion concentration, the green fluorescence of green fluorescent protein expression has been increasing, that is, the stimulation intensity of the zinc-sensitive promoter is different.
This proves that the zinc-sensitive promoter can work.
Working System Verification
Firstly, we synthesized the riboflavin synthesis gene (ribB) by direct DNA synthesis method. The riboflavin synthesis gene for riboflavin expression promotes electron transfer from the cell to the electrode and then produces current voltage changes.
Firstly, pzntR was ligated with ribB, and the ligated gene fragment was double digested with BamHⅠ and EcoRⅠ, and then inserted into plasmid pETDuet-1. The recombinant plasmid, named pETDuet-1pzntR-ribB, was transferred to Escherichia coli BL21 for expression.
We set up a two-compartment MFC-operated reactor with a working volume of 240 mL in each chamber, and the electrodes were pretreated before use. Carbon felt with an area of 16cm2 is used as an anode and cathode. These electrodes are connected via titanium wires to a 1000-ω external resistor.
In the MFC operating system, the anodic medium was supplemented with different concentrations (0-500μM) of Zn2+ in an M9 liquid medium for use by the Zn2+ response regulator. The cathode solution was potassium ferricyanide (100 mM ferricyanide in 50 mM phosphate buffer, pH 7.0), and the voltage was recorded at 10-min intervals in an MFC biosensor using a data acquisition device.
There is a significant linear relationship between zinc ion concentration and the maximum voltage of the constructed MFC biosensor.
The maximum voltage t-test of engineered bacteria and wild bacteria at 500μM zinc ion concentration showed that P < 0.001, indicating that riboflavin synthesized by ribB riboflavin synthesis gene could significantly promote electron transfer. This proves that our system works properly.
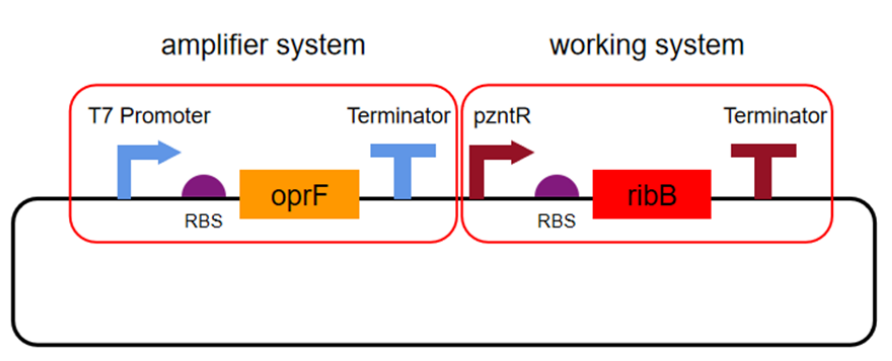
Amplification System Verification
RBS ribosome binding site
oprF porin gene
Terminator Terminator
pzntR zinc-sensitive promoter
RibB gene for riboflavin synthesis
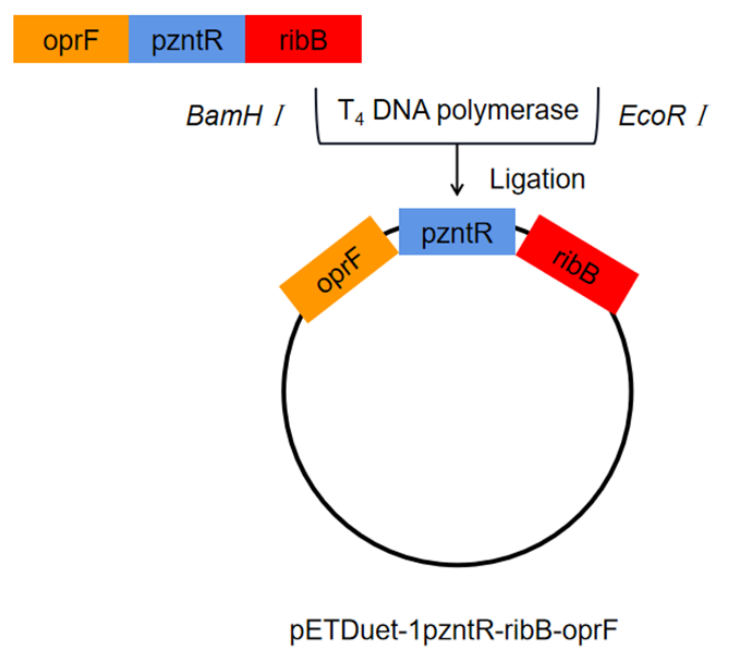
The porin gene (oprF) was synthesized by direct DNA synthesis. oprF and pzntR were ligated with ribB. The ligated gene fragment was double digested with BamHⅠ and EcoRⅠ and then inserted into plasmid pETDuet-1. The recombinant plasmid was named pETDuet-1pzntR-ribB-oprF, and the recombinant plasmid was transferred to E. coli BL21 for expression.
A two-compartment MFC-operated reactor was set up with a working volume of 240 mL in each chamber, and the electrodes were pretreated before use. Carbon felt with an area of 16cm2 is used as an anode and cathode. These electrodes are connected via titanium wires to a 1000-ω external resistor.
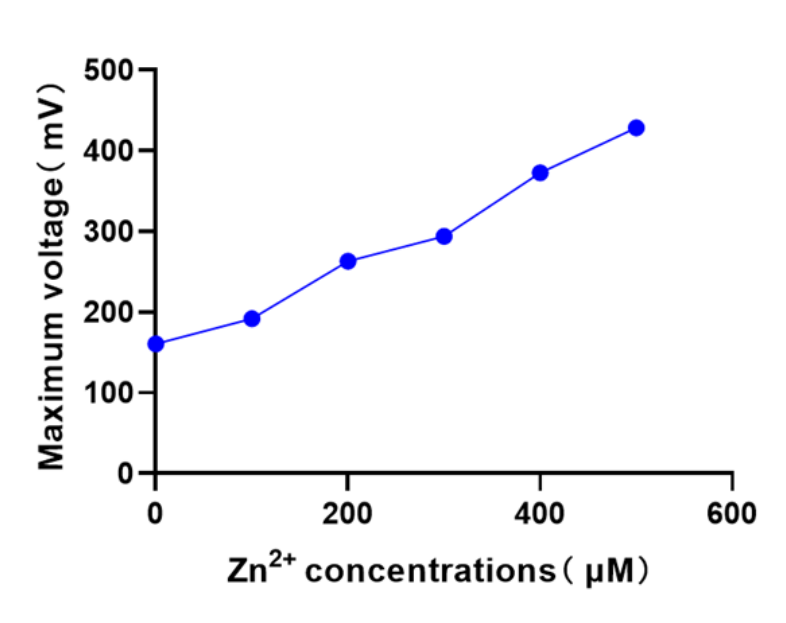
In the MFC operating system, the anodic medium was supplemented with different concentrations (0-500μM) of Zn2+ in the M9 liquid medium for use by the Zn2þ response regulator. The cathode solution was potassium ferricyanide (100 mM ferricyanide in 50 mM phosphate buffer, pH 7.0), and the voltage was recorded at 10-min intervals in an MFC biosensor using a data acquisition device.
There is a significant linear relationship between zinc ion concentration and the maximum voltage of the constructed MFC biosensor.
The expression of oprF in the engineered strain BL21 increased cell membrane permeability, and the highest voltage was approximately 4.5-fold higher than that of another engineered strain (pETDuet-1pzntR-ribB).
The two engineered bacteria were compared at the concentration of 500μM zinc ions The maximum voltage t-test of the engineered bacteria at the concentration of 500μM zinc ions showed P < 0.001, indicating that the oprF gene significantly changed the permeability of the cell membrane. So it turns out that the amplification system works.
pzntR+ ribB+ OprF
pzntR+ ribB Promoter sequence with recognition site for ZntR transcriptional regulator protein. ZntR activates transcription when Zn(II) is bound (1). ribB;the 3,4 dihydroxy-2-butanone-4-phosphate synthase; We get this part from E.coli genome by synthetize. It can increase the electric output by synthesis of riboflavin. Besides, riboflavin itself is yellow. After the addition of ribB, the bacteria will be yellow. The Microbial Fuel Cell (MFC) can be a future environmentally friendly biotechnological application for the production of electrical energy. As a future alternative energy source, the bioelectricity generation must become more efficient. A major limiting factor is the low bacterial membrane permeability, hindering transport of electron shuttles through the membrane and thereby restricting the electron shuttle-mediated extracellular electron transfer (EET) from bacteria to electrodes. This results in a reduced electrical power output of the MFC. Therefore, we heterologously expressed the porin protein OprF from Pseudomonas fluorescens into Escherichia coli.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 504
Illegal BglII site found at 1561 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 1953
Illegal AgeI site found at 394
Illegal AgeI site found at 436 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1474
| None |